
The modern world is filled with synthetic polymers, long-chained molecules designed by scientists to fill all manner of applications.
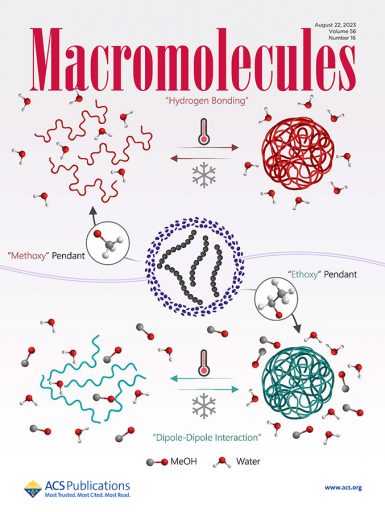
Researchers at FAMU-FSU College of Engineering have developed two closely related polymers that respond differently to high and low temperature thresholds, despite their similar design. The polymer pair could be used in applications in medicine, protein synthesis, protective coatings and other fields. Their work is published in Macromolecules.
“Typically, in order to have one thermal behavior, we have to prepare a polymer for that specific application, and if you want to have another extreme of polymer behavior, then you have to prepare a completely different polymer,” said coauthor Hoyong Chung, an associate professor in the FAMU-FSU College of Engineering. “But now, through this work, we have a single type of polymer that can be quickly adapted with minimal interference for both jobs.”
The researchers’ polymer is made with sulfoxide, a compound made of sulfur, oxygen and carbon molecules. One version contains an extra ingredient, a pair of hydrogen atoms known as a methylene group. This small structural variation is enough for each polymer to respond differently to variations in temperature.
Every mixture has critical temperatures above or below which the components will completely dissolve into a solution, regardless of the concentration of the various components in the mixture.
One version of the researchers’ polymer is soluble in water at low temperatures but becomes insoluble at higher temperatures. The other version displays the opposite behavior. It is insoluble at lower temperatures but dissolves when temperatures rise above a critical point.
“This contrasting behavior, which appeared with just a single minor change, was a surprising finding,” said postdoctoral researcher Biswajit Saha, the paper’s lead author. “It’s an exciting avenue for future research.”
Along with their development of this new, temperature-controllable polymer, the research team made other discoveries:
A new mechanism that governs a critical temperature threshold: Previous research showed that hydrogen atom bonds determined the temperature above which temperature-sensitive polymers dissolved in a solution, the so-called upper critical solution threshold. But Chung’s group found that the attraction between positively and negatively charged poles of different molecules — a process known as dipole-dipole interaction — also predicted the temperature at which their polymer would mix in water. Notably, his group has experimentally proved the presence of this interaction as a driving force of the thermal behavior.
Two-stage thermal behavior: Most solutions experience a single-phase change when they pass their temperature threshold. But the polymer developed by Chung’s team goes through phase changes in two stages. This feature could open potential new applications in medicine, such as a single medicine capsule that dissolves in the heat of a patient’s stomach in two stages, allowing for precise medicine delivery.
“We were fortunate to have these various insights with a single design,” Chung said. “A single polymer that can be ‘programmed’ to achieve different behaviors means this molecule can be easily adapted to different applications.”
This research was supported by the National Science Foundation.



