
Since the mid-1600s, chemists have been fascinated with brightly colored, coral-like structures that form by mixing metal salts in a small bottle.
Until now, researchers have been unable to model how these deceptively simple tubular structures —called chemical gardens — work and the patterns and rules that govern their formation.
In a paper published this week in the Proceedings of the National Academy of Sciences, Florida State University researchers lay out a model that explains how these structures grow upward, form different shapes and how they go from a flexible, self-healing material to a more brittle one.
“In a materials context, it’s very interesting,” said FSU Professor of Chemistry and Biochemistry Oliver Steinbock. “They don’t grow like crystals. A crystal has nice sharp corners and grows atom layer by atom layer. And when a hole occurs in a chemical garden, it’s self-healing. These are really early steps in learning how to make materials that can reconfigure and repair themselves.”
Typically, chemical gardens form when metal salt particles are put in a silicate solution. The dissolving salt reacts with the solution to create a semipermeable membrane that ejects upward in the solution, creating a biological-looking structure, similar to coral.
Scientists observed chemical gardens for the first time in 1646 and for years have been fascinated with their interesting formations. The chemistry is related to the formation of hydrothermal vents and the corrosion of steel surfaces where insoluble tubes can form.
“People realized these were peculiar things,” Steinbock said. “They have a very long history in chemistry. It became more like a demonstration experiment, but in the past 10-20 years, scientists became interested in them again.”
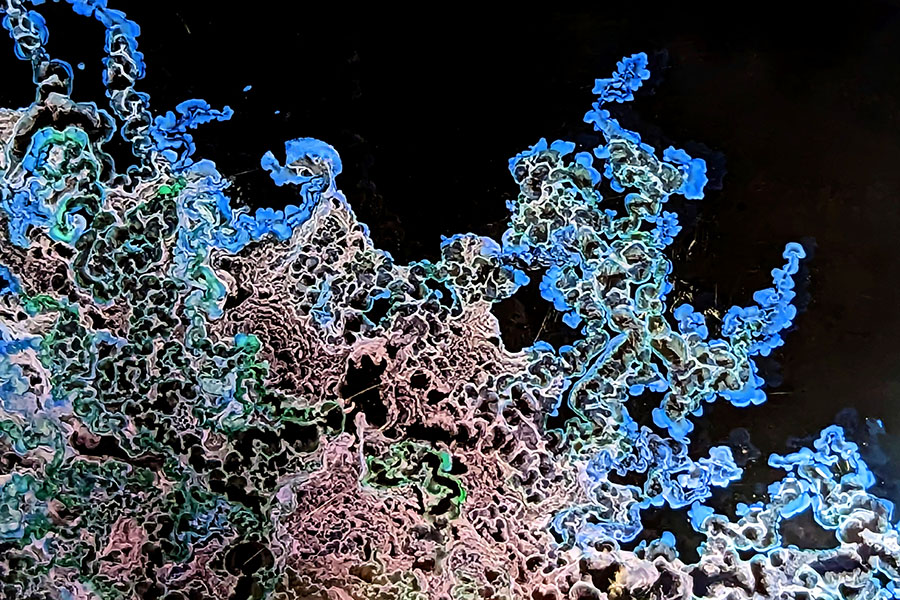
Inspiration for the mathematical model developed by Steinbock, along with postdoctoral researcher Bruno Batista and graduate student Amari Morris, came from experiments that steadily injected a salt solution into a larger volume of silicate solution between two horizontal plates. These showed distinct growth modes and that the material starts off as stretchy, but as it ages, the material becomes more rigid and tends to break.
The confinement between two layers allowed the researchers to simulate a number of different shape patterns, some looking like flowers, hair, spirals and worms.
In their model, the researchers described how these patterns emerge over the course of the chemical garden’s development. Salt solutions can vary a lot in chemical makeup, but their model explains the universality in formation.
For example, the patterns can consist of loose particles, folded membranes, or self-extending filaments. The model also validated observations that fresh membranes expand in response to microbreaches, demonstrating the material’s self-healing capabilities.
“The good thing we got is we got into the essence of what is needed to describe the shape and growth of chemical gardens,” Batista said.
To read the full paper, visit pnas.org. This work was supported by NASA and the National Science Foundation.




