
In the early days of the AIDS epidemic, a once-rare form of cancer known as Kaposi’s sarcoma (KS) emerged as a frequent harbinger of HIV. Its stigma was best illustrated by Tom Hanks, who portrayed a gay man trying to conceal the cancerous skin lesions from his co-workers in the 1993 movie “Philadelphia.”
A few years after the movie’s release, Fanxiu Zhu was a young virologist searching for a postdoctoral position. He found one, in Philadelphia, at a university laboratory investigating a newly identified virus linked to KS.
Today, Zhu is an assistant professor at Florida State University and a nationally recognized expert on KS. This spring, he won a new, five-year, $1.5 million grant from the National Institutes of Health (NIH) that will support the continuation of his widely published work, which could contribute to the development of more effective drug therapies.
“Kaposi’s sarcoma has become the defining symptom of AIDS, so studying the virus that causes KS is obviously important for understanding AIDS and AIDS-related cancers,” Zhu said.
Already Zhu’s body of research has generated findings that may speed the development of more effective, targeted therapies for KS, which is caused by a human cancer virus now known as human herpesvirus 8 (HHV-8) or KS-associated herpesvirus (KSHV).
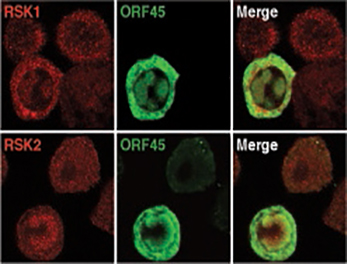
Zhu has become a leader in the study of KSHV by focusing on viral proteins that he and his Florida State research team have identified as crucial to the life cycle of the virus.
The protein they study extensively is called ORF45, short for “open reading frame 45.” Zhu and his team found that the ORF45 protein has a wide variety of functions in infection.
“In fact, Dr. Zhu has discovered that the protein is ‘packaged’ with each new virus, meaning that it is present when a virus infects a new cell,” said biologist P. Bryant Chase, chairman of FSU’s Department of Biological Science.
“Infectious agents and infected cells are typically recognized and then eliminated by our immune system,” Chase explained. “But Dr. Zhu’s group has shown that the KSHV ORF45 protein plays a role in preventing the immune system from recognizing infected cells and removing them.”
Gaining a better understanding of just how that happens is a key component of the new NIH grant.
An earlier study by Zhu’s research team found that the KSHV ORF45 protein “hijacks” the signaling pathways within cells that are critical for a cell’s regulation of its multiple processes — including what genes are expressed when and at what level, as well as what metabolic pathways will be turned on at any given time, and to what extent.
Now, Zhu’s latest grant will help him to clarify the KSHV ORF45 protein’s role not only within cellular signaling pathways but also in immune evasion — the process by which an invading organism avoids or suppresses the host’s immune responses to it.
Unlocking what once were the mysteries of the KSHV infection and replication are crucial to developing effective therapies because, despite the success of highly active antiretroviral therapy against HIV, KS remains a serious disease and the most common cancer among HIV/AIDS patients. KSHV also is associated with other human cancers such as primary effusion lymphoma and multicentric Castleman’s disease.
In Africa, KS accounts for nearly half of the reported cancers and is the leading cause of cancer death in some areas. Current treatments for KSHV-associated diseases are not optimal because they don’t attack the viral agent specifically.
“Dr. Zhu’s
work on KSHV ORF45 aims to give us a drug target that is specific to the virus,” Chase said. “The newly funded studies should take him even closer to that goal.”
Since 2008, nine papers coauthored by Zhu’s research team have been featured in leading peer-reviewed publications, including the Journal of Virology, PLoS Pathogens, Journal of Biological Chemistry and Journal of Immunology. To learn more, visit Zhu’s Florida State University Web page at www.bio.fsu.edu/faculty-zhu.
For additional information about Zhu’s research, visit the NIH website at this link.




