
Biological Science, Photo: Hui Jin, FSU Department of
Biological Science
Using yeast genetics and a novel scheme to selectively remove a single protein from the cell division process called meiosis, a cell biologist at The Florida State University has found that when a key molecular player known as Pds5 goes missing, chromosomes fail to segregate and pair up properly. Such failures in yeast result in sterility, and in humans, related errors can result in chromosomal mutations and birth defects such as Down syndrome.
That discovery is groundbreaking, but so, too, is what principal investigator Hong-Guo Yu calls the “genetics trick” performed by his research team that made the discovery possible. The study shines new light on the protein Pds5, its crucial regulatory role during meiosis, and the impact of its absence on the molecular-level genesis of human chromosomal birth defects that include Down, Edwards, Patau, Turner, Klinefelter’s and XYY syndromes.
The findings, which are described in a paper featured in the Journal of Cell Biology, may contribute to the eventual development of targeted, molecular-level interventions.
Yu, an assistant professor in FSU’s Department of Biological Science, explained how the meiotic stage is set and what goes wrong when key elements are rearranged.
“To produce a genetically balanced gamete (sperm and egg), the cell must contend with two sets of chromosome pairs, homologs and sisters,” he said. “Homologs are the nearly identical chromosomes inherited from each parent; sisters are exactly identical pairs that are produced like photocopies as part of normal cell division.
“During normal meiosis, the process of division that halves the number of chromosomes per cell, my colleagues and I discovered that Pds5 regulates the pairing and synapsis (joining together) of ‘mom and dad’ homologs. We also learned that Pds5 plays a vital role in the synaptonemal complex, a glue-like protein structure that homologs use to literally stick together as they pair up. In addition, we found that, although sister chromatids enter meiosis in very close proximity to one another, Pds5 acts to inhibit synapsis between them, a good thing because, then, meiotic conditions support the necessary pairing of homologs.”
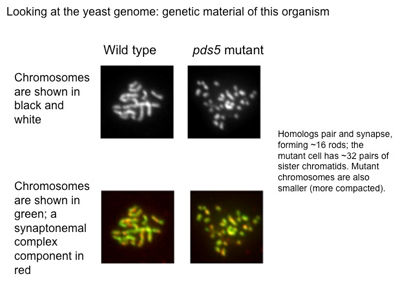
Consequently, removing Pds5 during meiosis triggers a chromosomal catastrophe.
“In order to observe what happened when the Pds5 went missing from the process, we performed a ‘molecular genetics trick’ that had never been applied to this particular protein before, and it worked,” Yu said. “We successfully engineered yeast cells that shut down Pds5 only during meiosis, but not when they were vegetative.”
As a result, Pds5 was no longer present to regulate homolog organization and transmission in the meiotic yeast cells. The synaptonemal complex, which normally would support the synapsis of homologs by creating a sticky bond along their entire length, failed to form. In the meiotic malfunction that followed, the identical sister chromosomes began to synapse instead.
“When Pds5 is removed and sister chromatids become synapsed as a result, the segregation and recombination of homologs essential for genetic diversity fails,” Yu said. “This finding is highly important, because failure to generate a crossover between homologs leads to chromosome missegregation and can cause human chromosomal birth defects such as Down syndrome, which affects about one in 800 newborns in the United States.”
Yu said the landmark study has significantly extended previous observations of the role of Pds5 in the formation of meiotic chromosome structure.
“Now, we are investigating the other factors that interact with Pds5 during meiosis to regulate chromosome segregation and homolog synapsis,” he said. “Long term, we hope to achieve a comprehensive understanding of the molecular mechanisms behind chromosomal birth defects and see our research contribute to the creation of targeted interventions during meiosis.”
Currently, Yu’s research at Florida State University is supported by a two-year, $150,000 Basil O’Connor Starter Scholar award from the March of Dimes Foundation, and by a three-year, $375,000 Bankhead Coley grant from the Florida Biomedical Research Program.
The Sept. 7, 2009, Journal of Cell Biology paper (“Pds5 is required for homologue pairing and inhibits synapsis of sister chromatids during yeast meiosis”) was co-authored by Hui Jin, a research technician in biology at Florida State, and Vincent Guacci, a postdoctoral assistant in the Department of Embryology at the Carnegie Institution of Washington.




