As the world’s premier winter athletes were preparing to take to the slopes, rinks and tracks for the 2018 Olympic Winter Games, Florida State University researchers were hard at work making a gold-medal discovery of their own.
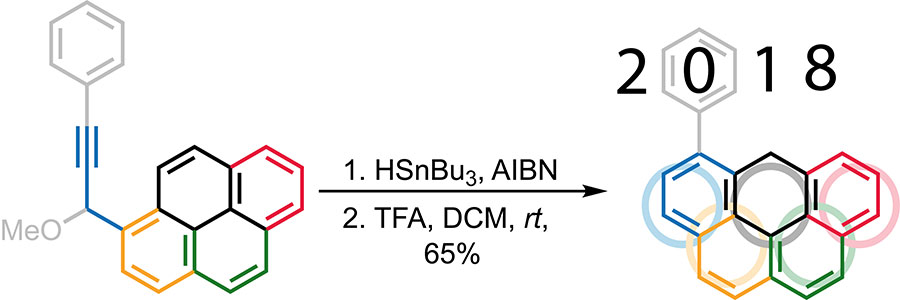
More than 7,000 miles away from the snowcapped peaks of PyeongChang, scientists from FSU’s Department of Chemistry and Biochemistry unlocked a novel strategy for synthesizing a highly versatile molecule called olympicene — a compound of carbon and hydrogen atoms named for its familiar shape.
“An olympicene is a molecule consisting of five rings that resemble the shape of the famous Olympic rings,” said Cottrell Family Professor of Chemistry and Biochemistry Igor Alabugin. “This new process for synthesizing these molecules offers a unique tool for the preparation of structurally precise carbon-rich nanostructures.”
The team’s findings were published in the journal Angewandte Chemie.
Olympicenes are like the decathletes of nanoscale molecules. Their range of potential applications include sophisticated sensors, information and energy storage, solar cells and high-tech LEDs.
The first olympicene molecule was unveiled by British chemists in anticipation of the 2012 London Olympics. Until now, synthesizing these unique structures was only possible through an arduous and intensive seven-step process based largely on chemistry dating back to the 1960s.
In the FSU team’s new technique, an additional hexagonal ring of carbon atoms is fused to the zigzag edge of an existing carbon-rich molecule in two quick steps.
Think plodding cross-country skier versus agile speed skater.
“Our success in developing this strategy allowed us to accomplish a two-step synthesis that is much shorter than the previously reported route, even though both methods used the same starting material,” Alabugin said.

It is olympicene’s relationship to graphene — a two-dimensional, single-layer lattice of carbon atoms — that may hold the most promise for these recognizably shaped molecules. Graphene is a world-beating material with truly Olympian properties: It is an efficient conductor of electricity and heat, it is almost completely transparent and, at 200 times stronger than steel, it is the mightiest material ever tested.
Soon after olympicenes were successfully synthesized, researchers recognized the important connection they shared with graphene. Now, with their new strategy for accelerated olympicene synthesis, Alabugin and his team may have revealed a way to better facilitate the production of what some have dubbed a “miracle material.”
“Our approach will allow chemists to synthesize a variety of olympicenes that can serve as stepping stones for the preparation of precisely shaped and functionalized graphene substructures,” Alabugin said.
In honor of this year’s Olympic Games, the team christened the product of their innovative synthesis strategy “Ph-olympicene,” — the “P” reflecting both the phenyl group crucial to the synthesis of the molecule and a subtle nod to the host city PyeongChang.
Alabugin said he considers the timing of his team’s discovery a rare, lucky moment of scientific serendipity.
“The exact timeline for designing, discovering and then having your findings peer reviewed is never certain,” he said. “Publishing this new synthesis of olympicene just in time for the Winter Olympics is indeed a fortunate coincidence.”
FSU researchers Nikolay Tsvetkov, Edgar Gonzalez-Rodriguez, Audrey Hughes, Gabriel dos Passos Gomes, Frankie White and Febin Kuriakose also contributed to the study. The research was supported by the National Science Foundation.




