Monoclonal antibodies, or mAbs, are important tools in medicine. These laboratory-made proteins are widely used in treatments for diseases such as cancer, Alzheimer’s disease, autoimmune disorders and infectious diseases.
New research from the Florida State University Department of Chemistry and Biochemistry shows that a particular type of monoclonal antibody known as NISTmAb retains its structure even if the sugar molecules attached to it are changed. The work, which was published in Chemical Communications, is a pivotal step in understanding protein dynamics in NISTmAb and offering clarity to biopharmaceutical developers who use monoclonal antibodies to develop new treatments.
“Understanding how sugar molecules influence how monoclonal antibodies behave has been a long-standing challenge in biotherapeutics,” said Associate Professor Christian Bleiholder, who led the research. “Modifying the sugar parts of the antibody doesn’t seem to affect its overall structure or how it behaves, which is important when developing antibodies as a treatment.”
Monoclonal antibodies are the largest group of biologic drugs, which are made from living organisms and come in many forms. These molecules need to maintain a specific structure to function properly, as any significant changes in their shape can disrupt their ability to bind to a target.
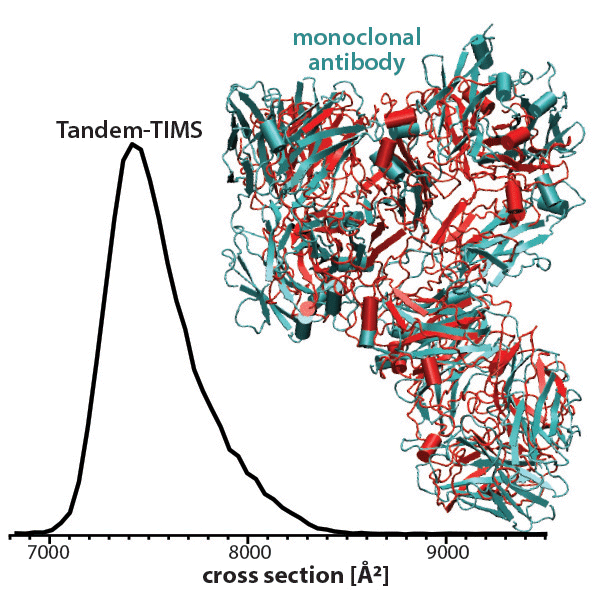
To study the mAb structure, Bleiholder worked with Melvin A. Park at Bruker Daltonics, to develop a groundbreaking technique called tandem-trapped ion mobility spectrometry, or Tandem-TIMS. The technique allowed scientists to study proteins and other molecules in a way that helps preserve their structure so researchers could study how their shape changes under different conditions.
This patented technology has been a decade in the making. Bleiholder learned how to perform ion mobility mass spectrometry as a postdoctoral researcher at the University of California, Santa Barbara, where he observed a molecular change during the onset of Alzheimer’s disease. When he started his laboratory in 2013 at Florida State University, he began the process of developing the new measurement technique. The result was a breakthrough that allowed scientists to investigate the structure and stability of complex proteins like NISTmAb in unprecedented detail.
“It was rewarding to see that after working for over 10 years on making this method we were finally able to apply it to solve problems,” Bleiholder said.
The study used Tandem-TIMS to isolate and analyze subpopulations of the NIST monoclonal antibody, a standard antibody in biotherapeutic research. Bleiholder’s team confirmed that structural variations among antibody populations are not influenced by glycosylation — a process in which sugar molecules attach to other molecules. The research marks a pivotal step in understanding protein dynamics and offering clarity to biopharmaceutical developers.
“Our study sheds new light on the relationship between the various structures of antibodies and their properties after protein translation,” said FSU faculty researcher Fanny C. Liu, who was the paper’s lead author. “This work opens exciting possibilities for accelerating the engineering of new biotherapeutic treatments for patients.”
The research will make it easier to design monoclonal antibodies by looking into how sugar molecules attached to them affect their shape and stability. This breakthrough could accelerate drug development timelines and enhance the quality of biotherapeutics, benefiting patients worldwide.
The research has sparked collaborations with industry leaders such as Johnson & Johnson to explore applications of this technology in enhancing biomedical development.
Bleiholder’s lab is expanding the applications of Tandem-TIMS to include the structural analysis of other proteins critical to biomedical research, including the SARS-CoV-2 spike protein and HIV capsid assembly. The lab is also pioneering methods for imaging proteins in tissue samples, paving the way for advances in personalized medicine.
FSU co-authors on this research were Jusung Lee and Thais Pedrete. Collaborators from Bruker Daltonics were Erin M. Panczyk and Stuart Pengelley. This work was supported by the National Science Foundation and the National Institutes of Health.



