
Researchers at the FAMU-FSU College of Engineering have made new discoveries on the effects of temperature on sustainable polymers. Their findings may help the industry to produce plastics that are better for the environment.
“Plastics made from petroleum, a non-renewable resource, remain too long in our land and water when discarded,” said Rufina Alamo, a professor in the Department of Chemical and Biomedical Engineering. “We are researching how sustainable polymers are heated and cooled so we may produce more ‘environmentally friendly’ plastics.”
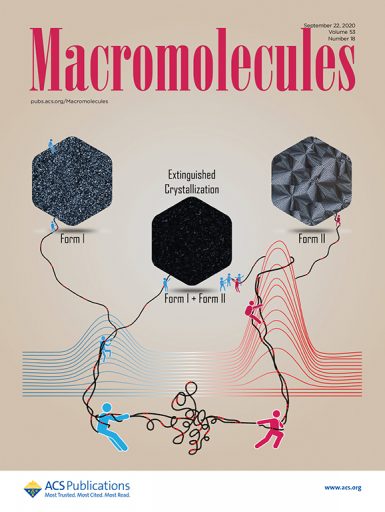
Alamo and former doctoral candidate Xiaoshi Zhang, now a postdoctoral research fellow at Penn State, recently published the work in a series of papers that focus on the crystallization of “green” polymers. The latest paper appears as the cover article in Macromolecules, a leading journal for polymer science.
“There is a worldwide motivation to transform how the largest volume of plastics are made,” Alamo said. “Polymer chemists and physicists are working hard to produce substitute materials to end problematic plastic waste.”
Determining the correct temperature for processing is key to producing better materials that will help scientists replace inexpensive polymers made from petroleum with economically viable, sustainable polymers.
“How the polymer is melted and cooled to make the desired shape is important,” Alamo said. “We are trying to understand the intricacies of crystallization to further understand the transformation process.”
The team is studying a type of polymer called “long-spaced polyacetals,” which are used in plastics. Synthesized in a laboratory at the University of Konstanz in Germany, the long-spaced polyacetals Alamo’s team used come from sustainable biomass. They contain a polyethylene backbone linked with acetal groups at precise equal distances. The structure combines the toughness of polyethylene with the hydrolytic degradability of the acetal group. This type of polymer is strong but breaks apart more easily with water than traditional polymers.
“What we discovered is these types of polymers crystalize in an unusual way when cooled after melting,” Alamo said.
During the cooling process, molecules that look like curly strands of spaghetti of melted plastics disentangle to form crystals and are responsible for the toughness of the final material. Alamo’s group showed that polymer crystallization is controlled by molecular events that take place at the crystal growth front.
The researchers found that when cooled rapidly, these polyacetals become tough and crystalline, and the molecules self-assemble in a type of crystal termed “Form I.” When cooled slowly, the material is also very crystalline, but the crystals formed are quite different and are dubbed “Form II.” When cooled at intermediate temperatures, the material does not solidify at all. This phenomenon has never been observed in any other crystalline polymers, according to the researchers.
“For crystals to be formed, an energy barrier first needs to be surmounted,” Alamo said. “At low temperatures, crystals are easily formed. At high temperatures, crystals are more stable, and at intermediate temperatures, the crystals compete to form, and the material can’t solidify.”
“This is a significant discovery because it is an important key to understanding how the plastics we use become solids,” she said. “We want to provide the industry with the best transformation processes possible. We want sustainable plastics that don’t warp or have difficulty solidifying.”
The research may provide new ways of manufacturing plastics that will be more economical to produce and sustainable.
Stephanie Marxsen from FSU, Patrick Ortmann and Stefan Mecking from the University of Konstanz, and Xiaobing Zuo from Argonne National Laboratory also contributed to this work, and undergraduate students, Sidney Cameron and Michael Parkhurst collected experimental data for the project.
This research is supported by a grant from the National Science Foundation.




